Nature of C-X Bond [Haloalkanes & Haloarenes]
NATURE OF C-X BOND :
• Halogens is more electronegative than carbons.
• Carbon atom has partial positive charge
• The nature of C-X bond depends on both the nature of carbon in the aromatic ring and the halogen attached.
• Florine has the highest electronegative element of halogens.
• The halogens elements(17th group) are just one electron short of completing their nearest novel gas configurations.
• Electronegativity increased across a period from left to right.
• The only one sigma bond is formed between one carbon and one halogen.
• The atomic size has increased Florine to down the period.
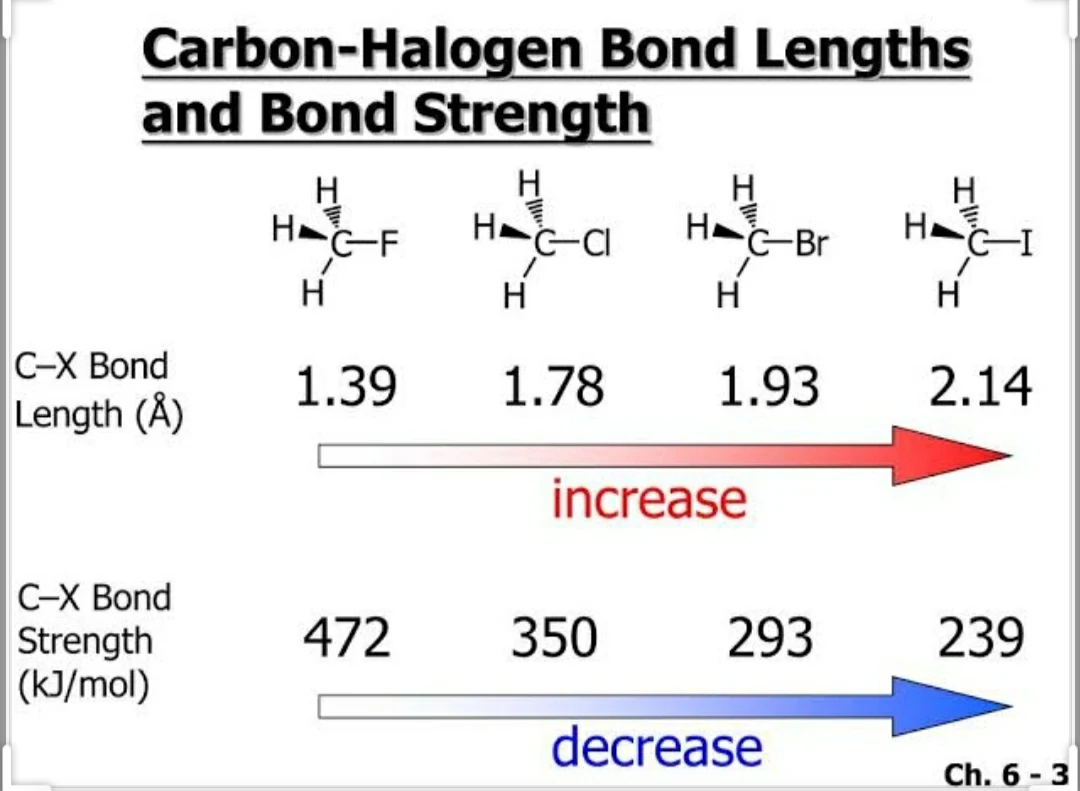
• The C-X bond length in haloarenes increases from Florine(F) to Astetine(As) and bond dissociated strength decreases.
• The bond polarity will decreases down the group of period.
Exception - C-Cl dipole moment is greater than C-F bond.









Comments
Post a Comment