Class 12th "Solutions" Chapter no. 02
RAOULT'S LAW :
Raoult's law is name after 'Francois Marie Raoult' who while conducting an experiment found out that when substances were mixed in a solution the vapour pressure of the solution decreased simultaneously. The law is established in the year of 1887.
"Raoult's law the states that a solvents partial vapour pressure in a solution is equal or identical to the vapour pressure of the pure solvent multiply by mole fraction in the solution."
Equation of Raoult's Law :
[ Psolution = Xsolvent × P°solvent ]
Where,
• Psolution = vapour pressure of solution.
• Xsolvent = mole fraction of solvent.
Calculation of Raoult's Law for Volatile Liquids :
For a solution of valatile liquids, the partial vapour pressure of each component in the solution is directly proportional to it's mole fractions.
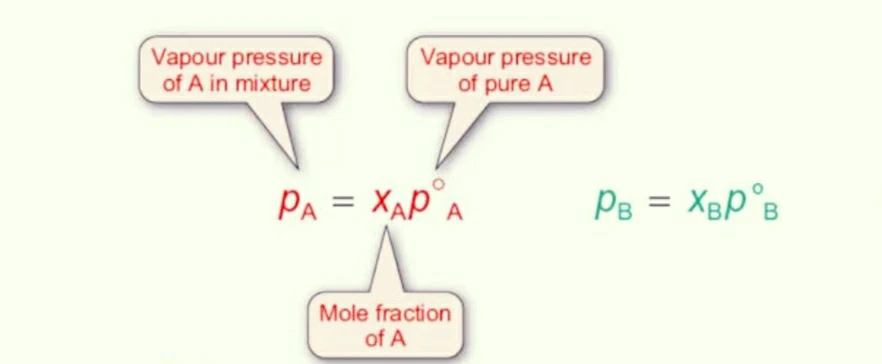
Example : If we consider as A & B volatile solution.
PA = P°A xA, PB = P°B xB
Where,
• PA = Partial pressure of A.
• P°A = Vapour pressure of pure A at that temperature.
• xA = Mole fraction of A in the liquid phase.
Similarly, PB = P°B xB
Hence, PT = PA + ....
PB (Dalton's law) = PA xA + ...
[ P°B xB = P°A + xB (P°B - P°A) ]
Importance of Raoult's Law :
• Raoult's Law is depends on the concentration of each (A & B) components in the liquid phase.
[ PA is proportional to XA, PB is proportional to XB = XA P°A = XB P°B ]
Limitations & Applications of Raoult's Law :
• It is describing ideal solution. However ideal solution are hard to find and they are rare.
• It is applicable to solutions containing non-volatile solutes only.
• It is not applicable to Solutes which dissociate or associate in the particular solutions.
• It is only for ideal solutions.
• It is only for very dilute solutions.
Ideal Solutions :
Practice Questions :
1. Is Raoult's Law is valid for all type of solutions. Explain it.
2. What is volatile & non-volatile solutions.
Thank you !




1: no. it doesn't apply on the all type of solution, it's only applyies on the ideal equation.
ReplyDelete2:Non-volatile substances exhibit a low vapor pressure and a high boiling point.
:A volatile substance is one that evaporates or sublimates at room temperature or below.
Well done!
ReplyDelete