Class 12th "SOLID STATE"
[A] Point Defects : The defects caused by a departure from the periodic arrangement of particles in the vicinity of an atom/group of atoms.
These are Three types of point defects :
{1} Stoichiometric defects : In this defects when crystal stoichiometry(e.g. in presence of +ve and -ve ratio in crystal) doesn't changed in the presence of crystal defects.
These are two important types of stoichiometric defects as follows :
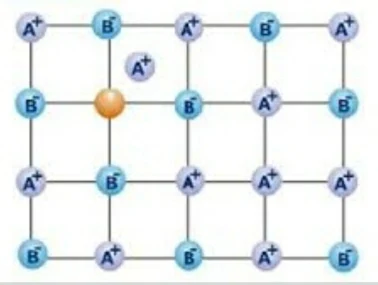
(i) Schottky Defects : This type of defect is created when one positive and one negative ion are missing equally from their positions. It is very common in ionic compounds which have high coordination number.
Note :
• Equal number of cations & anions should be missing for schottky Defects.
• It results decrease in density of crystal.
Example : NaCl, KCl, CsCl, KBr, AgBr.
It is also common in ionic compounds which have low coordination number.
It is also known as Dislocation defects.
Example : ZnS, AgCl, AgI, AgBr.
(Note : AgBr shown both defects due to small size of Ag+ ions).
Meaning - (Interstitial site : between the lattice point)
[ both the defects are most important topics from this chapter which would asked in board examination ]
THANK YOU !




Comments
Post a Comment