Class- 12th "Solid State"
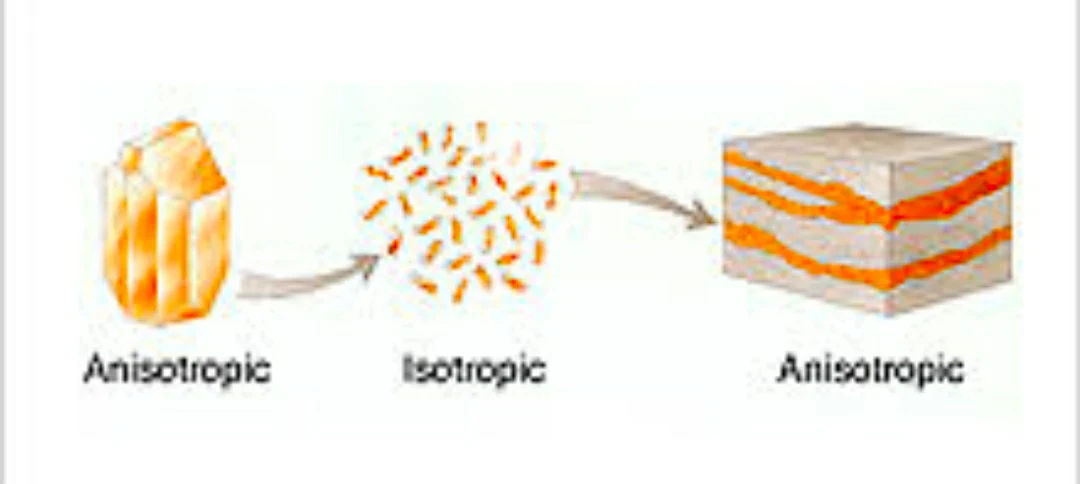
ANISOTRPOPY
It is the property of substance to show variations in physical properties along different moleculer axis. It is opposite of isotropic materials "अनिसोट्रॉपी एक सामग्री की संपत्ति है जो इसे अलग-अलग दिशाओं में अलग-अलग गुणों को बदलने या ग्रहण करने की अनुमति देती है जैसा कि आइसोट्रॉपी के विपरीत है। "

ALLOTROPES
When a substance/matter exists in two or more forms then they call allotropes.
Example : Diamond and graphite are allotropes of carbon.
POLYMORPHISM
When a single substance crystallized in two or more forms under different conditions of solidification is called polymorphism and the crystal showing polymorphism are called polymorphous.
Example : Silica forms many polymorphous such as alpha, bita- quartz, tridimite, crystobatic, coesite.
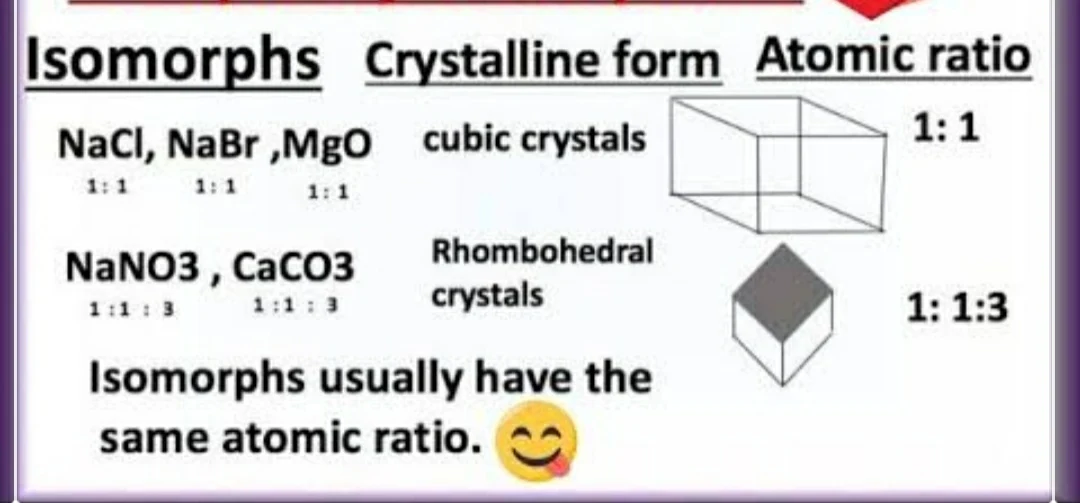
ISOMORPHISM
Iso = Same or identical
Morph = Shape or Forms
When two or more crystalline substances shows same crystalline structure is called isomorphim and which crystal shows this character is called isomorphous.
Example : K2SO4 and K2SeO4.
NaCl and KCl.
KClO4 and KMnO4.
THANK YOU !




Comments
Post a Comment